Medical Device CRO Market Size to Worth USD 19.9 Billion by 2034
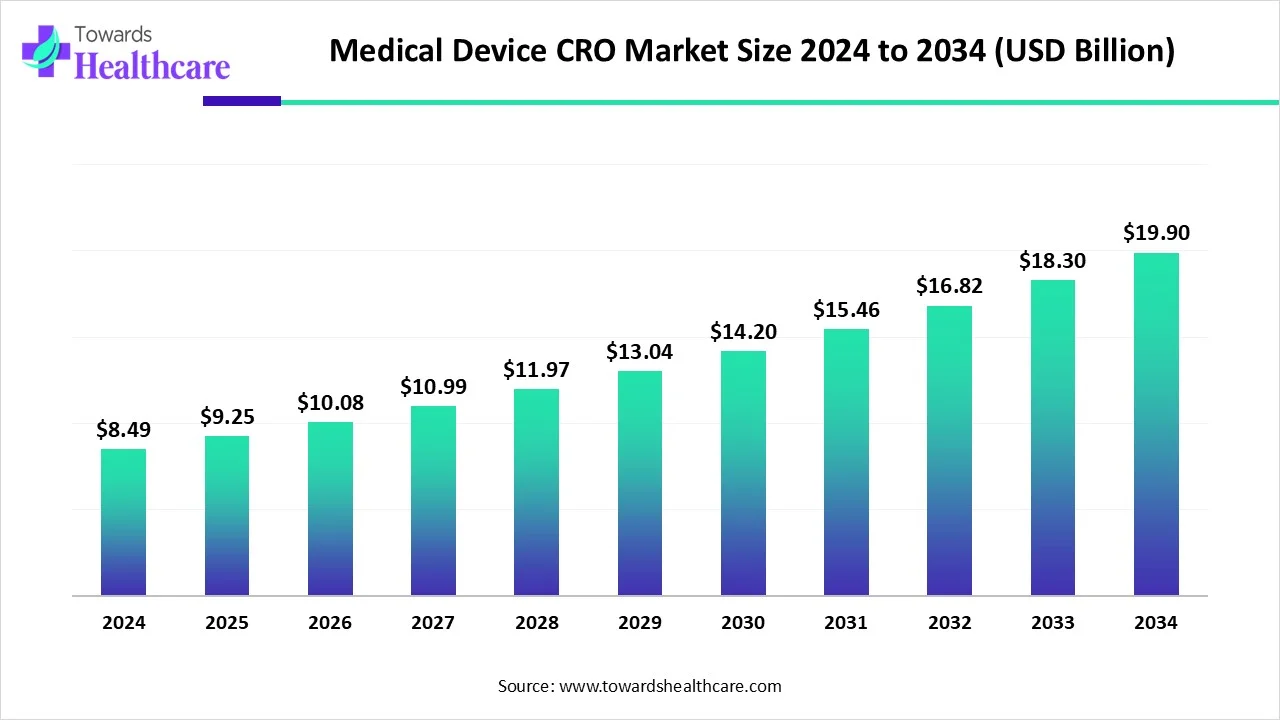
The global medical device CRO market size is calculated at USD 9.25 billion in 2025 and is expected to reach around USD 19.9 billion by 2034, growing at a CAGR of 8.98% for the forecasted period.
Ottawa, Sept. 25, 2025 (GLOBE NEWSWIRE) -- The global medical device CRO market size was valued at USD 8.49 billion in 2024 and is predicted to hit around USD 19.9 billion by 2034, rising at a 8.98% CAGR, a study published by Towards Healthcare a sister firm of Precedence Research.
Ongoing clinical trial pipelines and a widespread demand for highly sophisticated diagnostic devices, including wearables and RPM, are fueling the global market development.
The Complete Study is Now Available for Immediate Access | Download the Sample Pages of this Report @ https://www.towardshealthcare.com/download-sample/5712
Key Takeaways
- The medical device CRO market will likely exceed USD 8.49 billion by 2024.
- Valuation is projected to hit USD 19.9 billion by 2034.
- Estimated to grow at a CAGR of 8.98% from 2025 to 2034.
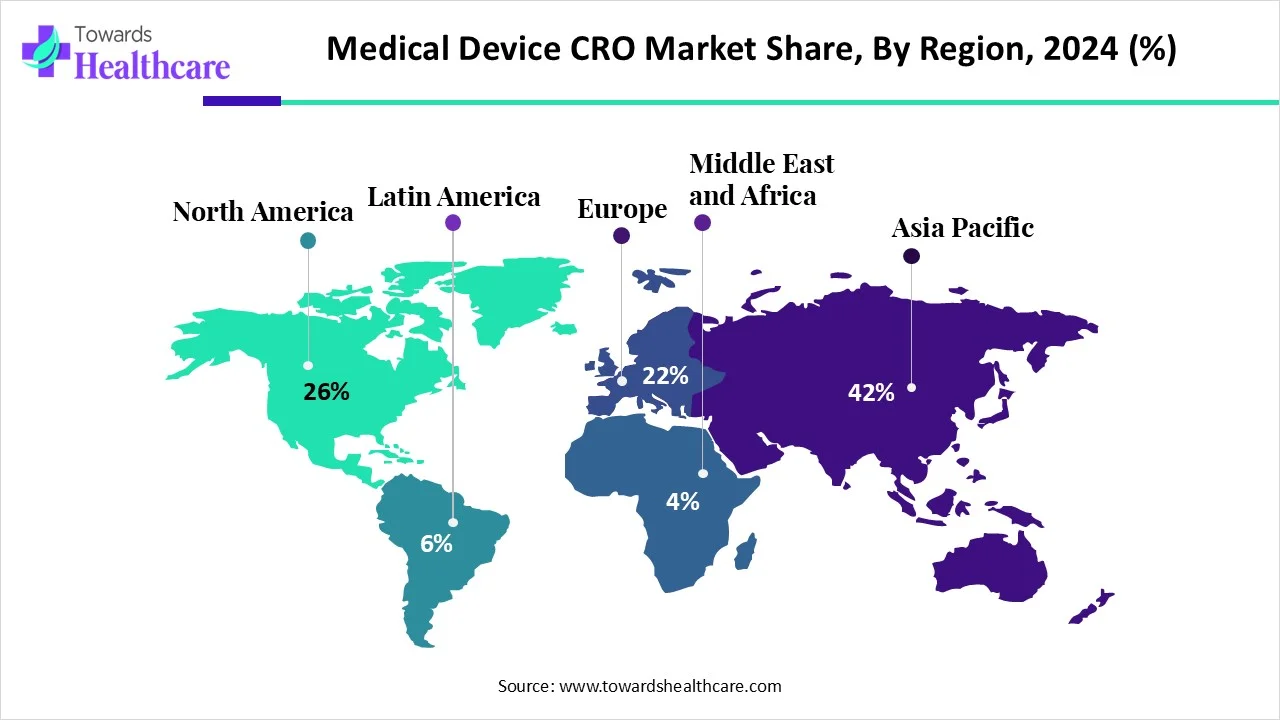
- Asia Pacific led the medical device CRO market share by 42% in 2024.
- North America is expected to be the fastest-growing region in the studied years.
- By phase, the clinical segment was dominant in the global market in 2024.
- By phase, the preclinical segment is expected to grow at a rapid CAGR in the predicted timeframe.
- By service, the clinical monitoring segment registered dominance in the market in 2024.
- By service, the regulatory/medical affairs segment is expected to witness rapid expansion during 2025-2034 in the global medical device CRO market.
- By device type, the diagnostic devices segment held a major share of the market in 2024.
- By device type, the MedTech devices segment is expected to grow rapidly during the forecast period.
What is the Medical Device CRO?
The global medical device CRO market encompasses a company that offers specialized services to medical device producers for their clinical trials, regulatory submissions, data management, and product development. Nowadays, the market is focusing on breakthroughs in noninvasive glucose monitoring, wearable infusion pumps, and novel sensors derived from materials, such as seaweed, that can monitor critical signs. Ongoing advances in robotic surgery support the development by integrating with FDA-cleared endovascular robots for procedures, including those used for spinal cord injuries.
Market Scope
| Metric | Details | |
| Market Size in 2025 | USD 9.25 Billion | |
| Projected Market Size in 2034 | USD 19.9 Billion | |
| CAGR (2025 - 2034) | 8.98 | % |
| Leading Region | Asia Pacific share by 26% | |
| Market Segmentation | By Phase, By Service, By Device Type, By Region | |
| Top Key Players | Avania B.V., Charles River Laboratories, CSSi Lifesciences, ICON plc., IQVIA, Labcorp Drug Development, Lindus Health, Medpace, NAMSA, Parexel, Syneos Health, WuXi AppTec | |
What are the Significant Drivers in the Medical Device CRO Market?
The global market is mainly fueled by the widespread benefits of CROs, as it lowers expenses, boosts speed-to-market timelines, and navigates stricter regulatory landscapes. Along with this, current growing investments in research and development (R&D) and accelerating complexity and innovation of medical devices, particularly digital and wearable technologies, are also impacting the overall market growth. As well as expanding innovation in diagnostics and implants and a rise in demand for specialized professionals is propelling the progress of CROs.
Become a valued research partner with us - https://www.towardshealthcare.com/schedule-meeting
What are the Key Trends in the Medical Device CRO Market?
The global market is stepping into the expansion of manufacturing facilities, advancements in novel devices, including robotic systems, and other advances in clinical trials.
- In September 2025, Governor Shapiro received $20 million investment from B. Braun to accelerate medical device manufacturing in the Lehigh Valley.
- In September 2025, the Medical Device Authority (MDA) of Malaysia and the Health Sciences Authority (HSA) of Singapore signed a Memorandum of Understanding (MOU) to strengthen regulatory cooperation and officially introduced a six-month pilot of the Medical Device Regulatory Reliance Program as part of the MOU.
- In September 2025, German healthcare company Siemens Healthineers and US-based medical device maker Stryker partnered to advance robotic systems for neurovascular interventions.
- In April 2025, HeartcoR Solutions, a leading ECG core lab, partnered with Wellysis, a leading digital healthcare company, for exclusive rights to the application of the Wellysis S-Patch device in clinical trials.
What is the Arising Challenge in the Medical Device CRO Market?
A major limitation in the global market is the requirement for robotic solutions to confirm compliance with data privacy regulations, as CROs handle and protect sensitive patient data. Ongoing intense competition is bolstering CROs to distinguish themselves through innovation, skilled personnel, and strategic planning to acquire and maintain a competitive edge.
Regional Analysis
Why did the Asia Pacific Dominate the Market in 2024?
In 2024, the Asia Pacific accounted for the biggest revenue share by 42% of the market. Due to the accelerating instances of diabetes, cancer, and cardiovascular concerns are fostering the medical device CRO market is growing in ASAP. Continuous alliances among ClinChoice and Medidata for clinical data management, NAMSA and Terumo for device development expansion. The emerging trend of outsourcing by medical device companies is exploring CRO expertise, propelled by the expanding complexity of devices and regulatory standards, along with a major rise in demand for home healthcare devices.
For instance,
- In January 2025, Yasuhiro Sensho, MD of Sensho Gumi, visited the YEIDA (Yamuna Expressway Industrial Development Authority) administrative office to foster partnership opportunities in the Medical Device Park (MDP) project.
You can place an order or ask any questions, please feel free to contact us at sales@towardshealthcare.com
Why did North America Grow Notably in the Market in 2024?
During 2025-2034, North America is anticipated to witness the rapid expansion in the medical device CRO market. This region’s market is increasingly involved in the development of the newest devices, like wearables and diagnostic tools, encouraging medical device companies to partner with CROs for specialized expertise and faster development. Furthermore, the FDA is leveraging standards and Good Clinical Practice (GCP) guidelines to support medical device companies in collaborating with CROs to ensure compliance.
For instance,
- In April 2025, ColdVentures, a leading medical device company, entered into a distribution agreement with PAL Medical, a Japan-based medical company specializing in advanced healthcare solutions, to introduce the advanced ColdVest technology to the Japanese market.
Segmental Insights
By phase analysis
How did the Clinical Segment Lead the Market in 2024?
The clinical segment held a dominant share of the medical device CRO market in 2024. Highly developed CROs are emerging methodologies that accelerate patient engagement and retention in clinical trials, further boosting their role and ultimately resulting in market growth. Alongside this phase is exploring technological integration, particularly AI/ML, remote monitoring through digital health and telemedicine, and patient-centric approaches like real-world data collection and user involvement. Additionally, diverse CROs are evolving deeper expertise in niche areas, such as oncology, rare diseases, and certain device types.
On the other hand, the preclinical segment is anticipated to expand rapidly in the coming era. Mainly, the emergence of advanced novel medical devices is required in advanced preclinical studies, which CROs can facilitate with cutting-edge technologies and infrastructure, resulting in highly effective and personalized research. Moreover, various preclinical tests are dependent on advanced techniques, such as in vitro and in vivo studies, computer simulations, and advanced cell models to assess device safety and efficacy. The globe is emphasizing revolution in biologics, cell and gene therapies are escalating the demand for specialized preclinical expertise and services.
By service analysis
What Made the Clinical Monitoring Segment Dominant in the Market in 2024?
The clinical monitoring segment held a major revenue share of the medical device CRO market in 2024. The implementation of hybrid monitoring solutions comprises a combination of targeted on-site source data verification (SDV) and remote reviews, with visit frequency adjusted based on site performance trends to lower on-site days. Comprehensive services encompass clinical trial design and management, regulatory consulting, data management, and post-market surveillance, to offer a simplified approach from concept to commercialization. Also, numerous CROs are combining AI and ML tools in the analysis of clinical trial data, allowing faster detection of safety concerns and protocol deviations.
However, the regulatory/medical affairs segment is estimated to witness the fastest growth. The wider contribution of regulatory bodies worldwide (e.g., FDA, EMA) has highly fostered stringent requirements for medical device approvals, requiring extensive documentation, clinical trials, and quality assessments. Emerging small and medium-sized enterprises (SMEs) and startups are often outsourcing regulatory affairs and clinical trial management to CROs to adopt affordability, leverage expertise, and emphasize core competencies.
By device type analysis
Why did the Diagnostic Devices Segment Lead the Market in 2024?
The diagnostic devices segment captured the dominating share of the medical device CRO market in 2024. A rise in global incidence of diseases, which accelerates demand for advanced diagnostics and CRO support, and the rising need for regulatory compliance and robust product development are driving the overall market progress. The broader population is highly demanding of remote and home-based diagnostics, which comprise at-home pregnancy tests, faster strep tests, and blood glucose monitors.
Onwards, the MedTech devices segment is predicted to register rapid growth. A prominent catalyst is the accelerating complexity and innovation of medical devices, as well as the growing demand for advanced devices, including wearables and diagnostics. Currently, the leading companies like Lindus Health, Medpace, ICON, Parexel, Labcorp, and Syneos Health are offering comprehensive services from early concept to post-market studies. Also, they use EDC systems and AI, which further boost clinical trials and the expansion of wearable devices and customized medicine.
Get the latest insights on life science industry segmentation with our Annual Membership: https://www.towardshealthcare.com/get-an-annual-membership
Recent Developments in the Medical Device CRO Market
- In July 2025, Veranex, a global provider of product development and contract research services, launched the industry’s first Innovation CRO (iCRO), an integrated development and research platform designed to move medical devices and in-vitro diagnostics (IVDs).
- In May 2025, Government-run Niloufer Hospital unveiled Amruth Swasth Bharath, an artificial intelligence (AI)-based diagnostic tool for non-invasive blood testing that delivers results in under a minute.
- In May 2025, Molecular Devices, LLC., a leading high-performance life science solutions provider, today launched the QPix FLEX Microbial Colony Picking System, which assists in automating and streamlining the multi-step microbial screening workflow, lowering manual labor and saving valuable time.
- In January 2025, Risklick, a spin-off from the University of Bern, introduced its latest product, Protocol AI, for medical devices, to expand the development of clinical trials while drastically lowering expenditures and risks.
- In March 2025, Johnson & Johnson (J&J) MedTech unveiled the Dualto Energy System, an electrosurgical generator that unites different energy modalities into an “integrated” platform for both open and minimally invasive surgery.
Medical Device CRO Market Key Players List
- Avania B.V.
- Charles River Laboratories
- CSSi Lifesciences
- ICON plc.
- IQVIA
- Labcorp Drug Development
- Lindus Health
- Medpace
- NAMSA
- Parexel
- Syneos Health
- WuXi AppTec
Browse More Insights of Towards Healthcare:
- The global smart medical devices market was valued at USD 24.82 billion in 2024, increased to USD 26.62 billion in 2025, and is projected to reach approximately USD 49 billion by 2034, expanding at a CAGR of 7.26% between 2025 and 2034.
- The global IoT medical devices market stood at USD 82.45 billion in 2024, rose to USD 105.54 billion in 2025, and is expected to hit nearly USD 971.27 billion by 2034, advancing at a robust CAGR of 28% during the forecast period.
- The global medical device contract manufacturing market was estimated at USD 78.61 billion in 2024, reached USD 87.14 billion in 2025, and is anticipated to climb to about USD 220.57 billion by 2034, growing at a CAGR of 10.86% from 2025 to 2034.
- The global medical device outsourcing market accounted for USD 160.3 billion in 2024, expanded to USD 180.59 billion in 2025, and is projected to achieve nearly USD 507.89 billion by 2034, with a CAGR of 12.65% over the forecast timeline.
- The global pain management devices market stood at USD 7.68 billion in 2024, grew to USD 8.41 billion in 2025, and is expected to reach approximately USD 19.1 billion by 2034, witnessing a CAGR of 9.54% between 2025 and 2034.
- The global medical device CRO market was valued at USD 8.49 billion in 2024, increased to USD 9.25 billion in 2025, and is anticipated to reach about USD 19.9 billion by 2034, recording a CAGR of 8.98% over the forecast period.
- The global wearable medical devices market totaled USD 42.78 billion in 2024, advanced to USD 53.68 billion in 2025, and is projected to soar to nearly USD 408.61 billion by 2034, expanding at a strong CAGR of 25.57% from 2025 to 2034.
- The global implantable medical devices market stood at USD 97.17 billion in 2024, rose to USD 103.14 billion in 2025, and is forecasted to reach nearly USD 176.33 billion by 2034, progressing at a CAGR of 6.14% during the study period.
- The global medical device testing market was valued at USD 10.77 billion in 2025 and is projected to reach approximately USD 24.32 billion by 2034, growing at a CAGR of 9.47% between 2025 and 2034.
- The global healthcare IoT security market was estimated at USD 0.62 billion in 2024, grew to USD 0.74 billion in 2025, and is anticipated to achieve around USD 3.52 billion by 2034, advancing at a CAGR of 18.83% from 2025 to 2034.
Segments Covered in the Report
By Phase
- Clinical
- Preclinical
By Service
- Clinical Monitoring
- Regulatory/Medical Affairs
- Project Management/Clinical Supply Management
- Data Management
- Medical Writing
- Quality Management/Assurance
- Bio-statistics
- Investigator Payments
- Laboratory
- Patient & Site Recruitment
- Technology
- Others
By Device Type
- Diagnostic Devices
- MedTech Devices
- Handheld Devices
- Others
By Region
- North America
- U.S.
- Canada
- Asia Pacific
- China
- Japan
- India
- South Korea
- Thailand
- Europe
- Germany
- UK
- France
- Italy
- Spain
- Sweden
- Denmark
- Norway
- Latin America
- Brazil
- Mexico
- Argentina
- Middle East and Africa (MEA)
- South Africa
- UAE
- Saudi Arabia
- Kuwait
Immediate Delivery Available | Buy This Premium Research @ https://www.towardshealthcare.com/price/5712
Access our exclusive, data-rich dashboard dedicated to the healthcare market - built specifically for decision-makers, strategists, and industry leaders. The dashboard features comprehensive statistical data, segment-wise market breakdowns, regional performance shares, detailed company profiles, annual updates, and much more. From market sizing to competitive intelligence, this powerful tool is one-stop solution to your gateway.
Access the Dashboard: https://www.towardshealthcare.com/access-dashboard
About Us
Towards Healthcare is a leading global provider of technological solutions, clinical research services, and advanced analytics, with a strong emphasis on life science research. Dedicated to advancing innovation in the life sciences sector, we build strategic partnerships that generate actionable insights and transformative breakthroughs. As a global strategy consulting firm, we empower life science leaders to gain a competitive edge, drive research excellence, and accelerate sustainable growth.
You can place an order or ask any questions, please feel free to contact us at sales@towardshealthcare.com
Europe Region: +44 778 256 0738
North America Region: +1 8044 4193 44
APAC Region: +91 9356 9282 04
Web: https://www.towardshealthcare.com
Our Trusted Data Partners
Precedence Research | Statifacts | Towards Packaging | Towards Automotive | Towards Food and Beverages | Towards Chemical and Materials | Towards Consumer Goods | Towards Dental | Towards EV Solutions | Nova One Advisor | Healthcare Webwire | Packaging Webwire | Automotive Webwire
Find us on social platforms: LinkedIn | Twitter | Instagram | Medium | Pinterest

Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.

